Abstract
Introduction: Initiation of hydroxyurea (HU) in the first year of life is becoming an increasingly accepted standard of care for children with sickle cell anemia (SCA). Stepwise dose escalation to maximum tolerated dose (MTD) typically starts empirically at 20 mg/kg/day, based on early studies of adults and the subsequent BABY HUG study. However, recent results from the NOHARM trial in Uganda demonstrated the clinical superiority and similar safety profile of a higher initial dose (25 mg/kg with dose escalation) compared to fixed 20 mg/kg dosing. Despite these findings, many providers remain hesitant to start at doses higher than 20 mg/kg/day, mostly related to possible hematologic toxicity. Similarly, there is great variability in dose escalation strategies and variation in laboratory thresholds used to decide whether to hold or dose escalate HU therapy.
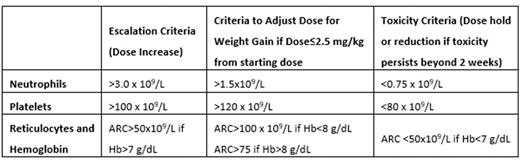
Study Design and Rationale: Recognizing the significant interpatient variability in dosing and the importance in optimizing exposure, we have developed an individualized, pharmacokinetics (PK)-guided dosing strategy of HU for children with SCA. The single center TREAT trial demonstrated that an individualized dosing strategy, using higher starting doses (27.7 ± 4.9 mg/kg/day) was feasible and effective, achieving MTD almost immediately and HbF ≥30% for most patients. To further validate these results, the follow-up Hydroxyurea Optimization through Precision Study (HOPS, NCT03789591) is a randomized, multicenter trial comparing standard, weight-based initial dosing with conventional dose-escalation vs. PK-guided initial dosing for children with SCD as they initiate HU therapy. Participants (ages 6 months through 21 years), recruited from 15 pediatric sickle cell centers across the US, are randomized to receive HU either using a starting dose of 20 mg/kg/day or a PK-guided dose. Participants in both arms subsequently use the same dose adjustment strategy, more aggressive than historical dosing strategies to optimize clinical response (See Table). The primary endpoint is clinical response defined by %HbF measured 6 months after starting HU.
As recruitment for the HOPS Trial is nearly complete but ongoing, we do not report primary or clinical results here. Here we demonstrate the feasibility of a PK-guided dosing across multiple centers and present data related to HU dosing and laboratory toxicity to demonstrate the safety of higher starting doses for young children with SCA.
Results: As of August 1, 2022, there have been 95, of a planned 104, participants enrolled in the HOPS Trial. The median age of enrollment was 10 months with most (72%) participants ≤ 2 years of age. Despite the young starting age, most participants had at least one complication or hospitalization related to SCA prior to starting HU, and 84% demonstrated significant hemolytic anemia, evidenced by a hemoglobin < 10.5 g/dL with absolute reticulocyte count > 150 x109/L prior to initiation of HU. The mean starting absolute neutrophil count (ANC) across this entire, mostly young cohort was relatively low (3.5 ± 2.2 x 109/L) with 52% of participants having a baseline ANC < 3.0x109/L and 24% with an ANC < 1.5 x 109/L prior to hydroxyurea initiation.
The starting doses were higher, as expected, for the PK-guided dosing arm (28.2 ± 5.2 mg/kg/day) compared to the weight-based dosing arm (20.2 ± 0.6 mg/kg/day). Despite high starting doses, there was no evidence of increased hematologic toxicity through the first 12 months of hydroxyurea therapy in the PK-guided dosing arm. To date, there have been a total of 24 dose holds in 18 participants due to hematologic toxicity, most commonly transient incidental neutropenia, with no differences between starting dose (i.e. higher or lower PK-guided dose) or study arm assignment (10 in PK-guided arm, 8 in weight-based arm). ANC was lower in the PK-guided dosing arm at month 6 compared to the weight-based dosing arm (2.2 ± 1.4 vs. 3.2 ± 1.9) but not in an unsafe range.
Conclusions: These preliminary results from the HOPS Trial demonstrate that starting doses of hydroxyurea as high as 25-30 mg/kg/day are safe and not associated with an increased risk of clinically significant hematologic toxicity in young children with SCA, even among young children with relatively low ANCs. Complete results of the HOPS trial are forthcoming. Which will formally evaluate the clinical benefits of PK-guided initial dosing compared to the traditional 20 mg/kg starting dose strategy.
Disclosures
Appiah-Kubi:Global Blood Therapeutics: Other: Commercial Advisory Board. Jacob:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Heeney:FORMA Therapeutics: Consultancy; Oric Pharmaceuticals: Consultancy; Novartis: Consultancy; Bluebird Bio: Consultancy; Vertex/ Crisper Therapeutics: Consultancy. Brown:Imara: Consultancy, Research Funding; Novo Nordisk: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy, Current Employment, Current equity holder in publicly-traded company, Research Funding; Forma Therapeutics: Research Funding. Meier:Global Blood Therapeutics: Current Employment. Piccone:Novartis: Speakers Bureau; Global Blood Therapeutics: Speakers Bureau. Quinn:Emmaus Medical: Research Funding; Aruvant: Research Funding; FORMA Therapeutics: Honoraria; Novo Nordisk: Honoraria; Dispersol Technologies: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal